Spring 2004
Molecule Interactions Problem Set
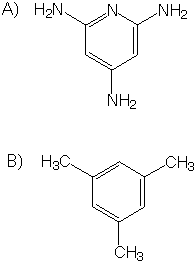
1) Which do you think has a higher boiling point, a neat (pure) solution of molecule A or a neat solution of molecule B?
molecule A
2) Which do you think has a higher boiling point, a neat solution of molecule B or a neat solution of methane?
molecule B
3) List the molecular interactions within neat liquids of A and of B. Give a one or two sentence description of each type of interaction.
Molecule A)
(i) short range repulsive interactions
The overlap of occupied orbitals of two atoms that are close together (i.e., separated by less than the sum of the van der Waals radii)
results in electrostatic repulsion. This repulsive interaction maintains the distance between molecules in a liquid at the sum of the van der Waals radii.
(ii) dipole interactions
The dipole in this molecule will interact with dipoles of nearby molecules (dipole-dipole interactions). These interactions are either attractive
or repulsive, depending on orientation. The dipole of one molecule will induce dipoles in nearby molecules (dipole-induced dipole interactions). These interactions
are always attractive.
(iii) fluctuating dipole (London Forces)
Molecules behave like oscillating dipoles. In molecules that are nearby to each other the oscillators couple. Coupled fluctuating dipoles engage
in favorable electrostatic interactions.
(iv) hydrogen bonding interactions
The nitrogen in the ring has a somewhat basic electron lone pair that would interact favorably with one or more of the acidic protons on the
exocyclic nitrogen donor atoms.
Molecule B)
(i) short range repulsive interactions
The overlap of occupied orbitals of two atoms that are close together (i.e., separated by less than the sum of the van der Waals radii) results
in electrostatic repulsion.
(ii) fluctuating dipole
Molecules behave like oscillating dipoles. In molecules that are located nearby to each other the oscillators couple. Coupled fluctuating dipoles
engage in favorable electrostatic interactions.
4) Give the answers below in a diagram. Estimate free energy (deltaG), the enthalpy (deltaH),
and the entropy contribution (TdeltaS) for transfer of one mole of each of the compounds A and B (above) from
i) vapor phase to neat liquid at 20 °C,
ii) neat liquid to dilute aqueous solution at 20 °C,
iii) vapor phase to neat liquid at 150 °C, and
iv) neat liquid to aqueous solution at 150 °C.
Answer for Molecule A
Answer for Molecule B
