Kitchen Chemistry. Acids and Bases, and a Red Cabbage Acid/Base Indicator
Loren Williams, School of Chemistry and Biochemistry, Georgia Tech (email: loren.Williams@chemistry.gatech.edu)
Experiment: Find out what is acidic and what is basic. Complete the following table by adding the correct solution to the vial with the acid/base indicator.
(see next page for background information)
| plus | color | acid or base? |
acid/base indicator | nothing | | neither (neutral) |
acid/base indicator | vinegar (acid) | | acidic |
acid/base indicator | windex window cleaner | | basic |
acid/base indicator | sodium bicarbonate (baking soda) | | |
acid/base indicator | lemon juice | | |
acid/base indicator | water | | |
acid/base indicator | Gatorade | | |
acid/base indicator | Seven up | | |
acid/base indicator | first a little vinegar, then windex | | |
acid/base indicator | first a little windex, then vinegar | | |
Questions. circle the answer
1) Is baking soda acidic or basic? acidic basic neither
2) Is lemon juice acidic or basic? acidic basic neither
3) Is water acidic or basic? acidic basic neither
4) Is Gatorade acidic or basic? acidic basic neither
5) Is Seven-up acidic or basic? acidic basic neither
6) Can you make acidic water into basic water? yes no maybe
7) Can you make basic water into acid water? yes no maybe
Background. The terms acidic and basic are very important to Chemists and Cooks. The words acidic and basic refer to amounts of hydrogen ions (H+) and hydronium ions (OH-) in water. Those ions affect how food tastes and changes as it is cooked. They are also important in many chemical reactions in our laboratory.
Acids. A glass of water with lots of H+ and not much OH- is acidic. A strong acid, like H2SO4 (sulfuric acid, found in car batteries), badly wants to break up into H+ and SO4-, and give away its H+. Hydrochloric acid, HCl, is another strong acid. So water that contains HCl is acidic. Very acidic water and strong acids like HCl are highly reactive and can be dangerous. But believe it or not, your stomach contains HCl and can be very, very acidic. You need the HCl in your stomach to help you digest your food. We are not going to use HCl in this experiment, because your stomach is made to handle strong acids but your skin and eyes are not.
Acids in the Kitchen. Weak acids can add a nice tart flavor to food, and can help clean up messes. One acid that you can probably find in your kitchen is called acetic acid (CH3COOH, vinegar). Vinegar is around 5% CH3COOH and 95% water. We will use CH3COOH and other weak edible acids in this experiment.
Bases. A solution with lots of OH- and not much H+ is basic. A strong base, like NaOH (lye, caustic soda) badly wants to break up into Na+ and OH- and give away its OH-. Very basic water and strong bases are highly reactive. For a long long time people have used a chemical reaction between NaOH and animal fat to make soap. Nearly 5000 years ago, in some of the human raceÕs first chemical reactions, people in Babylon made soap with that kind of chemical reaction. The ancient Egyptians also used NaOH to make soap. But the ancient Greeks did not seem to know how to run the chemical reaction and so did not have any soap. More recently NaOH (in Drano) is used to clean out clogged drains. We will not use NaOH in this experiment, because if you do not know how to handle it, you could burn your skin.
Bases in the Kitchen. A weaker base, that you use in your kitchen for cooking and cleaning and for making those little bubbles you see when you cook pancakes, is NaHCO3 (sodium bicarbonate, baking soda). We will use NaHCO3 in this experiment. One of the interesting things about NaHCO3 is that when you add it to acid (like CH3COOH from vinegar) the NaHCO3 is converted to H2CO3 (carbonic acid) which then falls apart into H2O (water) and CO2 (carbon dioxide). So when you add vinegar to baking soda, you can see bubbles of carbon dioxide.
Make Acids and Bases Disappear. If you add acid and base together, ZAP! they both disappear. Acid plus base gives water because H+ plus OH- equals H2O. So you can neutralize acid with base. And you can neutralize base with acid. That is why a base is sometimes called an antacid (an anti-acid). Your small intestine gives off NaHCO3 (base) to neutralize the HCl (acid) from your stomach.
Acid/Base Indicators. If you put an acid/base indicator in water, the water can have a very beautiful color. But the water is one color when you add acid and a different color when you add base. So you can use the color of an acid/base indicator to tell (to indicate) if the water is acidic or basic. You can get a useful acid/base indicator from red cabbage. If you cut up the cabbage and boil it, the acid/base indicator conveniently goes into the water. Just carefully pour the purple cabbage juice into a jar, leaving behind all the pieces of cabbage, and you have a lifetime supply of acid/base indicator. In this experiment we will use the acid/base indicator from red cabbage.
Other things to do.
1) Add acetic acid (vinegar) and sodium bicarbonate (baking soda) together. What happens?
2) Add acetic acid (vinegar) to uncooked egg white. What do you see? Try lemon juice instead of acetic acid. (Acid denatures (unfolds) the protein in the egg white).
3) Add acetic acid (vinegar) to milk. What do you see? Try the same thing with lemon juice (The acid denatures (unfolds) the protein in milk).
Additional Information. Molecules called anthocyanins are found in flower petals, leaves, blueberries, blackberries, strawberries, cranberries, cherries, apples and grapes, and in red cabbage. Anthocyanins cause the beautiful red/purple color that you see in roses and in the fall, in maple leaves. Anthocyanins are produced in leaves once chlorophyll (which is green) degenerates in the fall. Nature produces over 300 structurally distinct anthocyanins. By boiling red cabbage leaves, we can take out (extract) the anthocyanins. To get concentrated anthocyanins, cut a head of red cabbage into small pieces (about bite-sized) and boil for about 10 minutes in as little water as necessary to cover the cabbage. Slowly pour (decant) the anthocyanin solution into a jar and store it in the fridge. Anthocyanins dissolved in water (in aqueous solution) change color depending upon the amount of H+ and HO- in the water. (That is the same thing as saying that the absorbance of light by anthocyanins depends on pH.) Flowers such as hydrangea contain anthocyanins, and their color is sensitive to the acidity of the soil. The anthocyanins in red cabbage turn bright pink in acid solutions, reddish-purple in neutral solutions and green in basic solutions. But anthocyanins from other plants have other pH/color profiles. The effect of pH on blueberry anthocyanins is shown below.
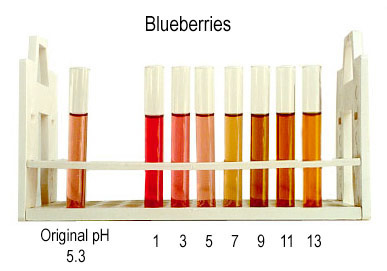
(from a web page at the University of British Columbia, Department of Agricultural Sciences.)
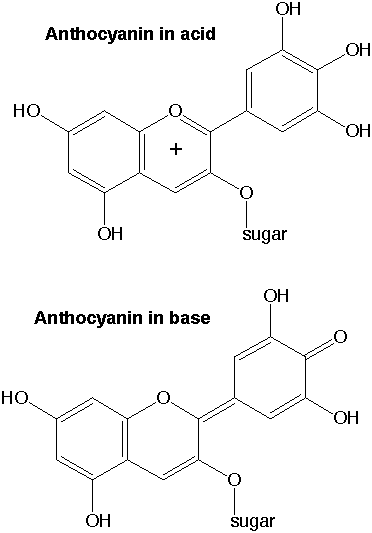
Each anthocyanin molecule contains a part that is made of sugar and a part that is made of polyphenol, as shown on the next figure.
Special Note to Teachers at Morningside Elementary School
If you would like to perform this or other chemistry demonstrations/experiments in your class, and would like assistance, either in the preparation phase or in the classroom, contact me (Loren Williams) at loren.williams@chemistry.gatech.edu / (404) 875-0390 (hm).